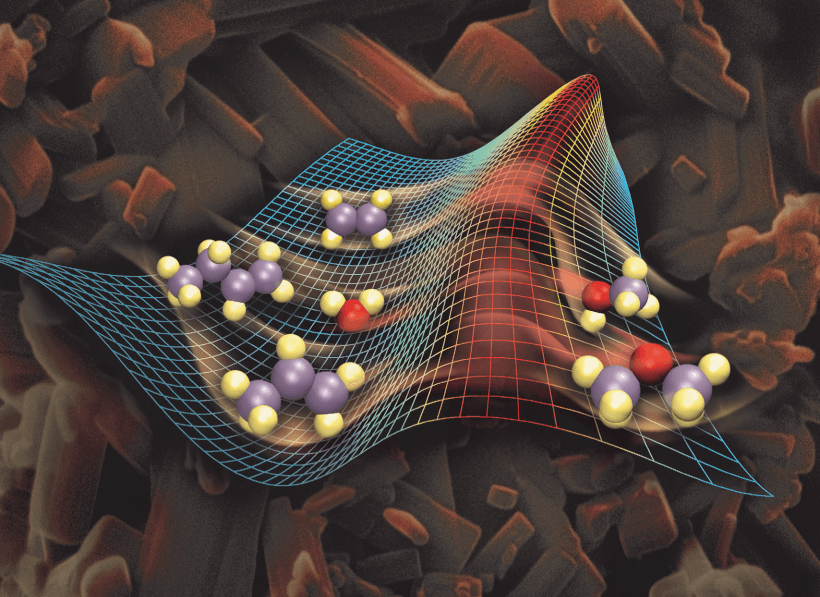
Catalysts are substances that accelerate chemical reactions without being consumed in the process. They play a crucial role in various industries, including pharmaceuticals, petrochemicals, and environmental protection. By lowering the activation energy required for a reaction to occur, catalysts enable the reaction to proceed faster and more efficiently.
One common example of a catalyst is platinum, which is used in catalytic converters to convert harmful pollutants in vehicle exhaust into less harmful substances. This helps reduce air pollution and improve air quality. Another example is enzymes, which act as biological catalysts in living organisms, facilitating essential biochemical reactions.
Catalysts can be classified into two main types: homogeneous catalysts, which are in the same phase as the reactants, and heterogeneous catalysts, which are in a different phase. Heterogeneous catalysts are often used in industrial processes due to their ease of separation from the reaction mixture.
The development of new catalysts is a vibrant area of research, with scientists constantly exploring novel materials and mechanisms to enhance catalytic efficiency. Catalyst design involves optimizing factors such as surface area, active sites, and selectivity to achieve desired reaction outcomes.
In addition to their role in chemical reactions, catalysts are also essential in fuel cells, where they facilitate the conversion of chemical energy into electrical energy. This technology holds promise for clean energy production and transportation.
Overall, catalysts are indispensable tools for driving chemical transformations and advancing various technological applications. Their ability to accelerate reactions and improve efficiency makes them invaluable in the quest for sustainable development and environmental stewardship.
I hope you find this content helpful and informative!